Last updated: February 18, 2022
*** For up-to-date information about the Omicron variant’s impact on COVID-19 therapeutics, refer to our variant clinical data summary.***
On this page:
- Introduction
- Drugs for Which There Is Evidence of Harm
- Other Drugs
- Table of Drugs Under Early Investigation for COVID-19
- Resources
- Multimedia
Introduction
A variety of therapeutics have been studied for treatment of COVID-19 for which there are varying degrees of evidence. For most, there are currently insufficient clinical data to recommend either for or against their use. For some, there is clear evidence of harm and no evidence of benefit. This overview is not a comprehensive summary, but includes therapeutics with strong biological plausibility that are available in the United States and are or will be studied by clinical trial.
Drugs for Which There Is Evidence of Harm
The following section addresses agents for which there is evidence of harm and no clear evidence of benefit for the treatment of COVID-19.
Hydroxychloroquine & Chloroquine
Overview
Hydroxychloroquine (HCQ) and chloroquine (CQ) are antimalarial agents that are also used to treat certain autoimmune disorders. HCQ has a lower incidence than CQ of adverse events with chronic use (Ben-Zvi, January 2011). Both drugs have immunomodulatory effects on various cytokines, including IL-1 and IL-6 (Ben-Zvi, January 2011), and prior to the COVID-19 pandemic were known to have in vitro effects against various viruses, including SARS (Keyaerts, October 2004; Vincent, August 2005; Savarino, September 2011). Early in the course of the pandemic both agents were also found to have in vitro activity against SARS-CoV-2 (Wang, February 2020; Liu, March 2020; Yao, March 2020). Given this potential biological plausibility, numerous clinical studies were initiated to examine efficacy for COVID-19 treatment.
In March 2020, the FDA issued an emergency use authorization (EUA) for the use of HCQ/CQ in hospitalized patients with COVID-19. In April 2020, the FDA released a statement cautioning against use of HCQ or CQ for COVID-19 outside of the hospital setting or a clinical trial due to risk of heart rhythm problems. Based on subsequent clinical trial data showing a lack of efficacy and concern for safety signals, the FDA revoked the EUA in June 2020 (see Safety section for data on mortality).
Since the beginning of the pandemic there has been extensive research related to HCQ, including more than five randomized controlled trials and several large observational cohort studies. Given the availability of higher-level data, in the following review we have focused on these studies. In non-hospitalized patients with asymptomatic or mild COVID-19, hydroxychloroquine has not been shown to reduce SARS-CoV-2 RNA viral load or disease progression in several small randomized trials (Skipper, October 2020; Mitja, July 2020). Similarly, a randomized double-blind, placebo-controlled trial of 821 participants in the United States found no impact on development of confirmed or probable COVID-19 when hydroxychloroquine was used as post-exposure prophylaxis within four days of a high-risk exposure (Boulware, August 2020).
Guidelines
- IDSA guidelines recommend against the use of hydroxychloroquinefor hospitalized patients with COVID-19.
- IDSA guidelines recommend against the use of hydroxychloroquineplus azithromycin for hospitalized patients with COVID-19.
- NIH guidelines recommend against the use of hydroxychloroquine and/or azithromycin for the treatment of COVID-19 in hospitalized and non-hospitalized patients.
Key Literature
In summary: While observational studies of the use of hydroxychloroquine (HCQ) and chloroquine (CQ) in patients with COVID-19 have had mixed results, several randomized controlled trials conducted in hospitalized patients with COVID-19 have not shown clinical benefit, nor have randomized controlled trials focused on post-exposure prophylaxis. In addition, some data suggest the use of HCQ may be associated with significant cardiac adverse events in patients with COVID-19. The use of HCQ or CQ is not recommended in patients with COVID-19.
Hydroxychloroquine in Hospitalized Patients with COVID-19 (The RECOVERY Collaborative Group, October 2020).
Overall, in this randomized, open-label trial, among patients hospitalized with COVID-19, those who received hydroxychloroquine did not have a lower incidence of death at 28 days compared with those who received standard of care.
Study population:
- Randomized, controlled, open-label platform trial comparing a range of possible treatments with usual care in patients hospitalized with COVID-19 in the U.K.
- 1,561 patients randomly assigned to receive hydroxychloroquine and 3,155 to receive standard of care.
- The mean age was 65.4 (SD ±15.3) years.
- A history of diabetes was present in 27% of patients, heart disease in 26% and chronic lung disease in 22%, with 57% having at least one major coexisting illness.
- At randomization, 17% were receiving invasive mechanical ventilation including extracorporeal membrane oxygenation, 60% were receiving oxygen only (with or without noninvasive ventilation) and 24% were receiving neither.
- The median duration of treatment was 6 days (interquartile range, 3-10 days).
Primary endpoint:
- 28-day mortality.
Key findings:
- Death at 28 days occurred in 421 of 1,561 patients (27%) in the hydroxychloroquine group and in 790 of 3,155 patients (25%) in the usual-care group (rate ratio, 1.09; 95% confidence interval [CI], 0.97 to 1.23; p=0.15).
- Patients in the hydroxychloroquine group had a longer duration of hospitalization than those in the usual-care group (median, 16 days vs. 13 days) and a lower probability of discharge alive within 28 days (59.6% vs. 62.9%; rate ratio, 0.90; 95% CI, 0.83 to 0.98).
- Patients in the hydroxychloroquine group had a greater risk of death from cardiac causes (mean [±SE] excess, 0.4±0.2 percentage points) and from non–SARS-CoV-2 infection (mean excess, 0.4±0.2 percentage points).
Limitations:
- Single-country study; patient population and results may not be generalizable to other countries.
- Physiologic, electrocardiographic, laboratory or virologic measurements were not collected.
Retrospective Cohort Study of HCQ +/-Azithromycin in Patients Hospitalized with COVID-19 (Arshad, July 2020).
Overall, in this retrospective cohort study, treatment with HCQ or HCQ + azithromycin was associated with reduction in COVID-19-associated mortality; however, the lack of balance between the groups and the lack of clarity around how treatment decisions were made limits the generalizability of these results.
Study Population:
- 2,541 hospitalized COVID-19 patients in Michigan.
- Patients received HCQ + azithromycin (783 patients), HCQ alone (1202 patients), azithromycin alone (147 patients) or neither drug (409 patients).
- HCQ + azithromycin were given only to select patients with severe COVID-19 and minimal cardiac risk factors.
- Corticosteroid use varied by group: HCQ, 78.9%; HCQ + azithromycin, 74.3%; azithromycin, 38.8%; neither medication, 35.7%.
- Mechanical ventilation was required in 13% of HCQ alone arm, 29% among HCQ + azithromycin, and in 8% that did not receive either medication.
Primary Endpoint:
- In-hospital mortality.
Key Findings:
- Overall in-hospital mortality was 18.1% (45% in ICU patients).
- Mortality by treatment category was as follows: HCQ + azithromycin, 157/783 (20.1% [95% CI: 17.3%–23.0%]), HCQ alone, 162/1202 (13.5% [95% CI: 11.6%–15.5%]), azithromycin alone, 33/147 (22.4% [95% CI: 16.0%–30.1%]) and neither drug, 108/409 (26.4% [95% CI: 22.2%–31.0%]).
- Compared to neither treatment, HCQ alone was associated with a 66% HR reduction (p < 0.001), while HCQ + azithromycin were associated with an HR reduction of 71% (p < 0.001).
- A matched propensity scoring of HCQ versus no HCQ (190 patients in each group) showed that HCQ compared to no HCQ was associated with decreased mortality by a mortality HR decrease of 51% (p<0.009).
Limitations:
- The retrospective nature of this study could have allowed for confounding.
- Determination of treatment decisions is not well described in the manuscript.
- Information on duration of symptoms prior to hospitalization is not available.
- There were substantial differences between the treatment arms with respect to corticosteroid use and their need for mechanical ventilation.
A Randomized Controlled Trial of HCQ in Outpatients With Early COVID-19 (Skipper, July 2020).
Overall, in this randomized controlled trial of outpatients with early COVID-19 disease, the use of HCQ was not associated with a change in symptom severity; adverse effects were common in the HCQ group compared to the placebo group.
Study population:
- 491 symptomatic, non-hospitalized adults with four days or less of symptoms and laboratory-confirmed COVID-19, probable COVID-19 or high-risk exposure within the preceding 14 days.
- 423 patients contributed to the primary endpoint: 241 (57%) health care workers, 106 (25%) household contacts and 76 (18%) with other exposures.
- Patients were randomized to receive HCQ or placebo.
Primary endpoint:
- Change in overall symptom severity over 14 days.
Results:
- Change in symptom severity over 14 days did not differ between the HCQ and placebo groups (difference in symptom severity: absolute, −0.27 points; P = 0.117).
- At 14 days, 24% (49/201) of participants receiving HCQ had ongoing symptoms compared with 30% (59/ 194) receiving placebo (P = 0.21).
- Medication adverse effects occurred in 43% (92/ 212) of participants receiving HCQ versus 22% (46/211) receiving placebo (P < 0.001).
- There was no difference in hospitalization (10 in the placebo group versus 4 in the HCQ group; P = 0.29).
Limitations:
- Participants were enrolled through internet-based surveys, which may have skewed the study population.
- The study population was heterogeneous.
- Only 58% of the sample were tested for SARS-CoV-2; it is possible not all the patients in the study had COVID-19.
Randomized Controlled Trial of HCQ in Patients with Mild to Moderate COVID-19 (Tang, May 2020).
Overall, in this randomized controlled trial that was halted early due to low enrollment, HCQ had no effect on the duration of viral detection, despite the use of high doses and a prolonged 2-3 duration.
Study Population:
- 150 hospitalized patients in China with laboratory-confirmed COVID-19.
- Illness severity at admission was primarily mild (15%) or moderate (84%); 1% of cases were severe.
- 75 patients were randomized to receive HCQ + standard of care, and 75 were randomized to receive standard of care. Study arm assignment was not blinded.
- The dose of HCQ used was 1,200 mg daily for 3 days, followed by 800 mg daily for 2-3 weeks.
- In comparison, the most used regimen in the United States is 800 mg on the first day followed by 400 mg daily for four days.
- The mean duration from symptom onset to randomization was 16.6 days.
Primary endpoint:
- Viral clearance by 28 days.
- Secondary endpoint: rate of improvement in symptoms over 1 month (defined as defervescence, improved oxygen saturation and disappearance of respiratory symptoms).
Key Findings:
- The probability of negative conversion by 28 days in the standard of care + HCQ group was 85.4% (95% CI: 73.8% to 93.8%), similar to that in the standard-of-care group (81.3%, CI: 71.2% to 89.6%).
- Adverse events were reported in 21/70 (30%) HCQ recipients versus 7/80 (9%) individuals not receiving HCQ.
Limitations:
- Blinding was not utilized during assignment to the study arms; selection bias is possible.
- The study was terminated early due to low enrollment and the impression of benefit (which may have led to underpowering).
- The time between symptom onset and randomization was 16.6 days; patients may have been too far along in the course of their illness to derive a beneficial anti-viral effect.
A Multicenter, Open-Label, Randomized, Placebo-Controlled Trial Evaluated Patients With Mild-to-moderate COVID-19 (Cavalcanti, July 2020).
Overall, in this open-label randomized clinical trial, in patients hospitalized with mild-to-moderate COVID-19, HCQ +/- azithromycin did not improve clinical status at 15 days compared to standard of care. Higher adverse events occurred in the patients who received HCQ +/- azithromycin.
Study population:
- 504 hospitalized patients with confirmed mild-to-moderate COVID-19 were randomized to receive either standard of care, HCQ + standard of care or HCQ + azithromycin + standard of care.
- There were 173 patients in the control group, 159 patients in the HCQ group and 172 patients in the HCQ + azithromycin group.
- The median time from symptom onset to randomization was 7 days.
Primary endpoint:
- Clinical status at 15 days using a seven-level ordinal scale.
Key findings:
- Compared to standard of care, the proportional odds of having a worse score on the ordinal scale at 15 days was not affected by either HCQ (OR 1.21; P=1.00) or HCQ + azithromycin (OR 0.99; P=1.00).
- Compared to standard of care, more adverse events were reported in patients that received HCQ + azithromycin (39.3%) or HCQ alone (33.7%).
- QTc interval prolongation and liver enzyme elevations were most common.
Limitations:
- The median time of symptom onset to randomization was 7 days, which limits the generalizability of these results.
- Blinding was not employed during randomization, which could have introduced several levels of bias.
- A good number of patients had already received HCQ and/or azithromycin prior to study entry, which could have affected the results.
RECOVERY, a Controlled, Open-Label Trial Comparing a Range of Possible Treatments in Patients Hospitalized with COVID-19 (Holby, July 2020).
Overall, in this randomized controlled open-label trial of patients hospitalized with COVID-19, HCQ was not associated with 28-day mortality reduction, but was associated with an increased length of hospital stay and increased risk of progressing to invasive mechanical ventilation or death.
Study population:
- 1561 patients were randomized to receive HCQ; 3155 patients were randomized to standard of care.
Primary endpoint:
- 28-day mortality.
Key findings:
- 28-day mortality did not differ in those patients that received HCQ vs. usual care (26.8% vs. 25.0% rate ratio 1.09; 95% CI 0.96 to 1.23).
- Patients allocated to HCQ were less likely to be discharged from hospital alive within 28 days (60.3% vs. 62.8%; rate ratio 0.92; 95% CI 0.85-0.99).
- Patients allocated to HCQ and not on invasive mechanical ventilation at baseline were more likely to reach the composite endpoint of invasive mechanical ventilation or death (29.8% vs. 26.5%; risk ratio 1.12; 95% CI 1.01-1.25).
Limitations:
- The mortality rate of patients with severe COVID-19 disease found in this study is higher than what has been generally found in this group in the United States.
- Open-label design; researchers and patients in the study knew who was receiving which treatment. This could have introduced bias into the results.
- Not all patients had proven SARS-CoV-2 infection via RT-PCR, but in post-hoc analysis of patients with a positive PCR result (90% of the sample), the results were similar.
Prospective Observational Study of HCQ in Hospitalized Patients With COVID-19 (Geleris, May 2020).
Overall, in this prospective observational study of patients hospitalized with COVID-19, HCQ was not associated with an increase or reduction in the composite endpoint of death or intubation.
Study Population:
- 1376 non-intubated patients hospitalized with COVID-19 at a single institution in New York City.
- 811 (58.9%) received HCQ; 45.8% were treated within 24 hours and 85.9% within 48 hours.
Primary endpoint:
- Composite of intubation or death in a time-to-event analysis.
Key findings:
- There was no significant association between HCQ use and intubation or death (HR 1.04, 95% CI 0.82-1.32).
Limitations:
- This is an observational study; therefore, confounding is possible.
- The decision to administer HCQ was made by the treating physician; selection bias may have occurred.
- The study occurred at a single institution; therefore, results may not be generalizable.
Randomized Controlled Trial of HCQ in Patients With COVID-19 (Chen, April 2020).
Overall, in this randomized controlled trial (RCT) of patients with mild COVID-19, HCQ was associated with a shorter time to clinical improvement (defined by resolution of fever and cough) and radiographic improvement. However, at study entry many patients had already met the time to clinical recovery study endpoints; it is not clear how the analysis accounted for these patients.
Study Population:
- 62 hospitalized patients with non-severe COVID-19.
- 31 patients were randomized to receive 5 days of HCQ, and 31 to receive standard of care.
- Standard of care included antiviral agents, antibacterial agents and immunoglobulin with or without corticosteroids.
Primary Endpoint:
- Time to clinical recovery (defined as becoming afebrile and cough relief for 72 hours or more), clinical characteristics and changes in chest CT.
Key Findings:
- Compared with the control group, body temperature recovery time was significantly shortened in the HCQ treatment group (2.2 ± 0.4 days vs 3.2 ± 1.3 days).
- Cough duration was shorter in the HCQ group compared to the control group (2.0 ± 0.2 days vs 3.1 ± 1.5 days).
- More patients had radiographic improvement with HCQ [25/31 (81%) vs. 17/31 (55%), p=0.05].
Limitations:
- No baseline characteristics of patients were provided.
- The endpoint focused on resolution of fever and cough; the clinical relevance of this endpoint is not clear.
- At entry into the study, in the HCQ arm nine patients had no fever and nine had no cough. In the control arm 14 patients had no fever, and 16 had no cough. It is not clear how these patients were accounted for in the analysis.
- No information about viral load was included.
Safety Concerns
Across the body of evidence from several RCTs, treatment with HCQ may increase the risk of experiencing adverse events (Tang, May 2020, Cavalcanti, July 2020). One RCT suggests increased risk of QT prolongation among patients treated with HCQ+AZ compared to those not receiving HCQ (RR: 8.50; 95% CI: 1.16-62.31) (Cavalcanti, July 2020). Early reports raised concern about the association of hydroxychloroquine with in-hospital mortality. A systematic review and meta-analysis (Fiolet, August 2020) of the effect of hydroxychloroquine with or without azithromycin on the mortality of patients with COVID-19 found that hydroxychloroquine alone was not associated with any mortality impact (RR = 1.09 [95% CI 0.97-1.24, n = 3 studies] for randomized controlled trials), but that the combination of hydroxychloroquine and azithromycin was associated with a significant increase in mortality (RR = 1.27; 95% CI 1.04-1.54, n = 7 studies). Because of this finding, many clinical studies, including the European DisCoVeRy clinical trial or the WHO international Solidarity clinical trial, discontinued all treatment arms with hydroxychloroquine.
Ivermectin
Overview
Ivermectin is an antiparasitic agent that is FDA-approved for use in animals and humans to treat/prevent certain parasitic infections, such as those caused by helminths and scabies. Ivermectin is one of a class of drugs, called avermectins, which are naturally produced by soil-dwelling Streptomyces species and have broad antiparasitic properties. Ivermectin is not authorized or approved by the FDA to prevent or treat COVID-19. The standard dose of ivermectin is unlikely to inhibit replication of SARS-CoV-2 in humans, and current clinical trial evidence is insufficient to support the use of ivermectin to treat patients with COVID-19.
At high doses, ivermectin is associated with gastrointestinal symptoms such as nausea, vomiting and diarrhea; hypotension; and neurologic effects such as decreased consciousness, confusion, hallucinations, seizures, coma and death. Early in the pandemic in April 2020, FDA issued a warning that formulations of ivermectin intended for veterinary use should not be repurposed and used in humans. Ivermectin may increase the effects of other drugs that cause central nervous system depression such as benzodiazepines and barbiturates, according to an August 2021 CDC Health Alert warning against the use of ivermectin to prevent or treat COVID-19. The advisory noted that U.S. prescriptions of ivermectin had increased 24-fold from March 2019 to August 2021, and that there has been a parallel increase in the number of calls to poison control centers relating to adverse effects from ivermectin.
Current recommendations note there is insufficient evidence to recommend using ivermectin in patients with COVID-19 outside of a clinical trial. Ongoing clinical trials are examining the use of ivermectin in COVID-19.
Guidelines
IDSA guidelines suggest against the use of ivermectin outside of a clinical trial and summarize the evidence for the use of ivermectin in patients with COVID-19.
- Inpatients: Evidence from randomized controlled trials failed to show that ivermectin reduced or increased mortality among hospitalized persons with COVID-19 (RR: 0.66; 95% CI: 0.31, 1.42; low CoE).
- Outpatients: Ivermectin had no effect on mortality, progression to severe disease or viral clearance at 7 days (RR: 0.48; 95% CI: 0.13, 1.76; very low CoE, RR: 0.64; 95% CI: 0.26, 1.54; very low CoE, and RR: 1.13; 95% CI: 0.79, 1.62; very low CoE, respectively).
NIH guidelines note there is insufficient evidence to recommend for or against the use of ivermectin for the treatment of COVID-19. These guidelines also provide an in-depth review of relevant clinical studies related to ivermectin and COVID-19.
Key Literature
While some have suggested ivermectin can inhibit the replication of SARS-CoV-2, to reach the plasma concentration equivalent to that needed to achieve an in vitro antiviral effect, doses of up to 50-100-fold higher than the maximum approved dose in humans (200 μg/kg once a day) would be required (Schmith, October 2020). A separate modeling study found that ivermectin is unlikely to reach the proposed inhibitory concentration (IC50) in the lungs after oral administration of either the approved dose or at doses 10x greater than the approved doses as a single dose (Jermain, December 2020).
Some evidence suggests ivermectin may have anti-inflammatory or immunomodulatory effects in other clinical scenarios, such as allergic asthma and bacterial infection, and some have suggested that its proposed effect in late-stage inflammatory COVID could have more to do with these properties than its antiviral effect (Yan, June 2011; Ci, August 2009; Zhang, November 2008). The impact of ivermectin on antiviral immune response has not been explored.
Current clinical trial evidence is insufficient to support the use of ivermectin to treat patients with COVID-19. A systematic review and meta-analysis found that, compared with standard of care or placebo, ivermectin did not reduce all-cause mortality, length of stay or viral clearance in people with COVID-19. Several ongoing prospective, randomized, controlled clinical trials (including the ACTIV-6 study, the COVID-OUT study, and the PRINCIPLE trial) are examining the use of ivermectin in COVID-19. The ivermectin arm (N= 677 in ivermectin and 678 in placebo arms) of the large, randomized TOGETHER trial was stopped for futility, after finding that the proportion of participants requiring hospitalization was 86/677 for the ivermectin group and 95/678 for the placebo group, for a relative risk of hospitalization of 0.91 (0.69-1.19); mortality was also not significantly impacted (RR 0.82 [0.44-1.52]) (Reis/Mills presentation, August 2021 – PDF).
Safety Concerns
When used for the approved indications, at the currently approved doses, ivermectin is a safe drug. Although ivermectin has a wide margin of safety, toxicity can occur, and the risks of toxicity increase when it is used at higher than approved dosages. Adverse effects of ivermectin overdose include gastrointestinal symptoms such as nausea, vomiting and diarrhea.
Ivermectin toxicity is partially mediated by cross-targeting of GABA-gated chlorine channels in the human central nervous system, may also relate to mdr-1 gene variants, and typically manifests with CNS side effects such as depression, coma and death (Geary, November 2005; Temple, October 2021; Chandler, February 2018). Overdoses are also associated with hypotension as well as gastrointestinal symptoms such as nausea, vomiting and diarrhea. Ivermectin may increase the effects of other drugs that cause central nervous system depression such as benzodiazepines and barbiturates.
CDC has issued a Health Advisory warning against the use of ivermectin to prevent or treat COVID-19 and FDA has issued a warning cautioning against human use of ivermectin veterinary products due to safety and toxicity considerations. There have been numerous cases reported to U.S. poison control centers of significant adverse events after self-administration of ivermectin for the prevention or treatment of COVID-19.
Other Drugs
Fluvoxamine
Fluvoxamine is a selective serotonin reuptake inhibitor approved by the FDA since 1994 and used in the treatment of depression (now available at low cost as a generic). Unlike other drugs of its class, fluvoxamine stimulates sigma-1 receptors on the surface of the endoplasmic reticulum, which processes and traffics proteins within cells. This results in dampened inflammatory response to sepsis in laboratory animals, and has also been shown to block SARS-CoV-2 replication (Hashimoto, March 2021). In this way it can be thought of as having both immunomodulatory and antiviral properties. Other hypothesized mechanisms of action of fluvoxamine against SARS-CoV-2 include its inhibition of platelet activation and its targeting of lysosomes (Homolak, August 2020; Schlienger, May 2003). Of note, fluvoxamine is an inhibitor of certain cytochrome P450 metabolizing enzymes, including CYP3A4, CYP1A2 and CYP2C9, so may increase levels of any co-administered drug that is metabolized by those enzymatic pathways.
Because of these theoretical, in vitro, and preclinical benefits, fluvoxamine (50 mg twice daily for 14 days after a loading dose of 50-100 mg) was administered to a cohort of horse track employees who developed COVID-19 in a California outbreak related to shared congregate living conditions. This prospective cohort study found that all 65 recipients of fluvoxamine had resolved their disease symptoms at the end of 14 days, whereas 29 of the 48 people who declined treatment still had residual symptoms at 14 days (and six were hospitalized, two intubated, and one died) (Seftel, February 2021). Subsequently, fluvoxamine (100 mg 3x daily for 14 days) was studied against placebo in a preliminary small-scale study in St. Louis, MO of 152 non-hospitalized individuals with PCR-confirmed COVID-19, and a significantly lower percentage of the fluvoxamine group had clinical deterioration at 15 days than the placebo group (0% as compared to 8.3%, respectively; 95% CI 1.8% to 16.4%; log-rank p=0.009) (Lenze, December 2020). Overall, these studies generated the hypothesis that fluvoxamine may reduce hospitalizations for adult outpatients with symptomatic COVID-19.
A much larger recent randomized platform trial in Brazil, called the TOGETHER trial, was released as a preprint in late August 2021, showing similar preliminary findings. The trial randomized symptomatic adults with confirmed COVID-19 to receive either fluvoxamine (100 mg PO BID x 10 days) or placebo; the primary endpoint was extended observation in the Emergency Department for COVID-19 or hospitalization for COVID-19. A total of 739 participants received fluvoxamine and 733 received placebo. There was an observed lower rate of hospitalization in the fluvoxamine (10.4%) than in the placebo group (14.7%) (ITT RR 0.71; 95% Bayesian Credible Interval 0.54-0.93), with a 99.4% posterior probability that fluvoxamine was superior. The trial was stopped by its data and safety monitoring board for superiority on August 6, 2021. Final data that encompasses all days of follow-up for all participants will be forthcoming.
WHO Solidarity PLUS Trial Agents
In late 2021, the World Health Organization plans to launch a new phase of its Solidarity PLUS trial in 52 countries to test three candidate drugs: artesunate (an FDA-approved antimalarial used in intravenous form for the treatment of severe malaria), infliximab (an FDA-approved monoclonal antibody which blocks TNF alpha) and imatinib (an FDA-approved signal transduction inhibitor/tyrosine kinase inhibitor used for the treatment of Philadelphia chromosome positive leukemias).
Artesunate
Artesunate, like imatinib, may have both antiviral and immunomodulatory effects in COVID-19. These are theorized to be due to its inhibition of endocytosis of virus, NF-kappa B mediated dampening of cytokines such as TNF-alpha, IL-6, and IL-1, and its inhibition of MMP-2 and MMP-9 (Magenta, December 2014; Xu, February 2007). It has been shown in vitro in green monkey kidney Vero E6 cells to have anti-SARS-CoV-2 activity with clinically achievable EC50 values of 12.98 ± 5.30μM (Cao, July 2020). A clinical study of 43 patients with COVID-19 reported statistically improved clinical outcomes in the artesunate group as compared to the control group (Lin, April 2020). Some have hypothesized that artesunate might have beneficial effects in mitigating the neurological complications of COVID-19.
Regarding route of dosing, it is important to note that, while the Solidarity PLUS trial will use intravenous artesunate, the oral and intravenous forms of artesunate have been shown to have equivalent efficacy and tolerability in other settings, such as the treatment of Plasmodium falciparum malaria (Alin, December 1995). Infliximab will be also given intravenously, and imatinib orally.
Infliximab
The background for the choice of these three drugs is complex. It has been observed in several settings that patients receiving various types of immune inhibitors have attenuated cases of COVID-19. The SECURE-BID registry found that patients with IBD on anti-TNF antibodies had lower risk of death or hospital admission than those not on these agents (aOR 0.60 [95% CI 0.38-0.96], p=0.03) (Brenner, August 2020). The COVID-19 Global Rheumatology Alliance registry likewise found that patients with rheumatologic disease on anti-TNF agents +/- other immunomodulators had lower rates of hospital admissions (aOR 0.40 [95% CI 0.19-0.80], p=0.01) (Gianfrancesco, July 2020). These observations, combined with the moderate success of other cytokine-blocking agents (e.g. tocilizumab), have led to the study of infliximab in a broader patient population hospitalized with COVID-19. A small (n= 17) open-label phase 2 study (NCT04425538) of infliximab in patients with COVID-19 has completed enrollment and results are forthcoming. The CATALYST phase 2 randomized adaptive trial of N=146 patients with COVID-19 pneumonia in the UK compared infiliximab to either namilumab (a granulocyte-macrophage colony-stimulating factor inhibitor) or standard of care, for reducing inflammation as measured by C-reactive protein (CRP). The study found no benefit from infliximab and a high rate of adverse events in the infliximab group (narilumab, however, did appear to significantly reduce inflammation in hospitalized patients) (Fisher, June 2021 – preprint, not peer-reviewed).
Imatinib
Regarding imatinib, some mechanistic studies have noted that the SARS-CoV and possibly SARS-CoV-2 viruses rely on ABL2 kinases to infect host cells, and therefore that blocking this kinase specifically might have antiviral activity (Sisk, May 2018; Coleman, September 2016). Efforts to identify potentially useful antiviral drugs from large-scale high-throughput screening identified imatinib as a leading anti-SARS-CoV-2 contender, along with mycophenolic acid and quinacrine dihydrochloride (Han, January 2021). It has also been suggested that imatinib may exert its effects partially as an immunomodulator (i.e., not as a direct antiviral) via unclear mechanisms. Indeed, imatinib has been shown to have very low potency against SARS-CoV in Vero E6 cell cultures, with a clinically unachievable EC50, and in ACE2+ human Caco-2 cells, had no inhibitory effect (Zhao, November 2020). Some early clinical data suggested that imatinib may stop pulmonary capillary leak and confer clinical benefit to patients hospitalized with COVID-19 (Aman, June 2021). Case reports of rapid improvement of severe COVID-19 exist (Ortega, September 2020). A randomized placebo-controlled study of imatinib among 385 participants hospitalized with COVID-19 found a lower 28-day mortality in the imatinib group than in the placebo group (HR 0.51 [0.27-0.95]), but when factors associated with mortality were controlled for, aHR was non-significant at 0.52 (95% CI 0.26-1.05). There was however a significantly shorter duration of mechanical ventilation requirement in the imatinib group than the placebo group (median duration 7 days [3–13] vs. 12 days [IQR 6–20] respectively; p=0·0080), and shorter length of intensive care unit stay in the imatinib group (median duration 8 days [5–13] vs. 15 days [7–21] in the placebo group; p=0·025). Notably, 276 of the 385 study participants (72%) also received dexamethasone.
Other Drugs
Using high-throughput methods of identifying potential compounds of promise for the treatment of SARS-CoV-2, several drugs have been identified, many of which have entered clinical trials. Compounds that are hypothesized to block the binding of ACE2 and the receptor-binding domain include nobiletin, glycyrrhizin, neohesperidin and SSAA09E2. Antiprotozoal agents such as nitazoxanide and ivermectin have been identified with AI and simulation-based methods as having some interaction with protein targets on SARS-CoV-2, and are theorized to have anti-inflammatory effects. Several “naturally occurring” compounds have been shown to inhibit SARS-CoV-2 in vitro (resveratrol, ginkgolic acid, baicalein) and also to synergistically augment antiviral effect when used in combination with existing antivirals (linoleic acid + remdesivir; cepharanthine plus nelfinavir) (Yang, June 2021).
Other direct antivirals (other than remdesivir) include nucleoside/nucleotide analogues, such as favipiravir (an RdRp blocker that has approval in Japan for the treatment of influenza and is being studied for COVID-19). A recent systematic review and meta-analysis of clinical trials of favipiravir for COVID-19 (with a total of 1,019 participants) found that there was a significantly higher likelihood of clinical improvement in favipiravir recipients than in control recipients in the 14 days after hospitalization ((OR = 3.03, 95% CI = 1.17–7.80), but no significant improvement in mortality. Other RdRp inhibitors include ribavirin, galidesivir and molnupiravir (note: updated information to come following Merck announcement of interim phase III results via press release). Inhibitors of viral protease enzyme, including disulfiram, lopinavir-ritonavir, darunavir, N3, 11a/11b and carmofur, are also of some interest as antivirals against SARS-CoV-2.
TMPRSS2 inhibitors such as camostat mesylate block SARS-CoV-2 cell entry in vitro and have been theorized to block SARS-CoV-2 entry into lung tissue in COVID-19 pneumonia, but a double-blind randomized, placebo-controlled trial in N=205 hospitalized patients with COVID-19 pneumonia failed to show any impact of camostat on mortality (HR 0.82 [95% CI 0.24-2.79; p=0.75]) or on time to clinical improvement (Gunst, May 2021). A related and more potent TMPRSS2 inhibitor, nafamostat, is being investigated in COVID-19 patients in a subsequent randomized placebo-controlled clinical trial (the RACONA study) which opened in June 2021.
As a general rule, in the human immune response to viruses, interferon-mediated responses precede pro-inflammatory responses (Galani, January 2021); this rule does not apply to SARS-CoV-2, where patients with severe COVID-19 had delayed and muted IFN production but robust pro-inflammatory cytokines. However, it has been observed that higher IFN-ɣ responses correlate with lower bronchial SARS-CoV-2 viral load and improved outcomes, and that lower IFN-ɣ is associated with lung fibrosis in COVID-19 patients (Hu, September 2020). In keeping with this observation, interferons have also been considered in the treatment of COVID-19, with the COVIFERON randomized controlled trial of IFNβ1a and IFNβ1b in patients with severe COVID-19 showing a significant difference in time to clinical improvement in the IFNβ1a arm as compared to the control group (HR 2.36[95% CI 1.10-5.17], p=0.031) (Darazam, April 2021). There was an extremely high mortality in all three arms (20%, 30% and 45% in the IFNβ1a, IFNβ1b and control arms, respectively).
Various other host-targeted therapeutics have been clinically tested for COVID-19, with the hope that they will prevent the dysregulated inflammatory response and their efficacy will be unchanged regardless of variant. Opaganib is a new oral drug which selectively inhibits sphingosine kinase 2 (SK2), which is an essential component of the body’s signaling response to Tumor Necrosis Factor, leading to the inflammatory cascade. It also has in vitro antiviral activity against a variety of viruses, and has been employed in five patients as treatment of severe COVID-19 under a compassionate use program in Israel, with reportedly salutary effects when compared to matched controls, including lower CRP, faster resolution of lymphocyte count, and comparatively more disease resolution (Kurd, November 2020). It was then studied in a phase 2 randomized, placebo-controlled trial in 40 adults with COVID-19 pneumonia in the U.S., and there was a greater proportion of patients on opaganib who were weaned to room air on day 14 than on placebo (53% as compared to 22%; no statistics shown in the poster presented at the 2021 World Microbe Forum). A larger phase 2/3 study is underway, with results pending.
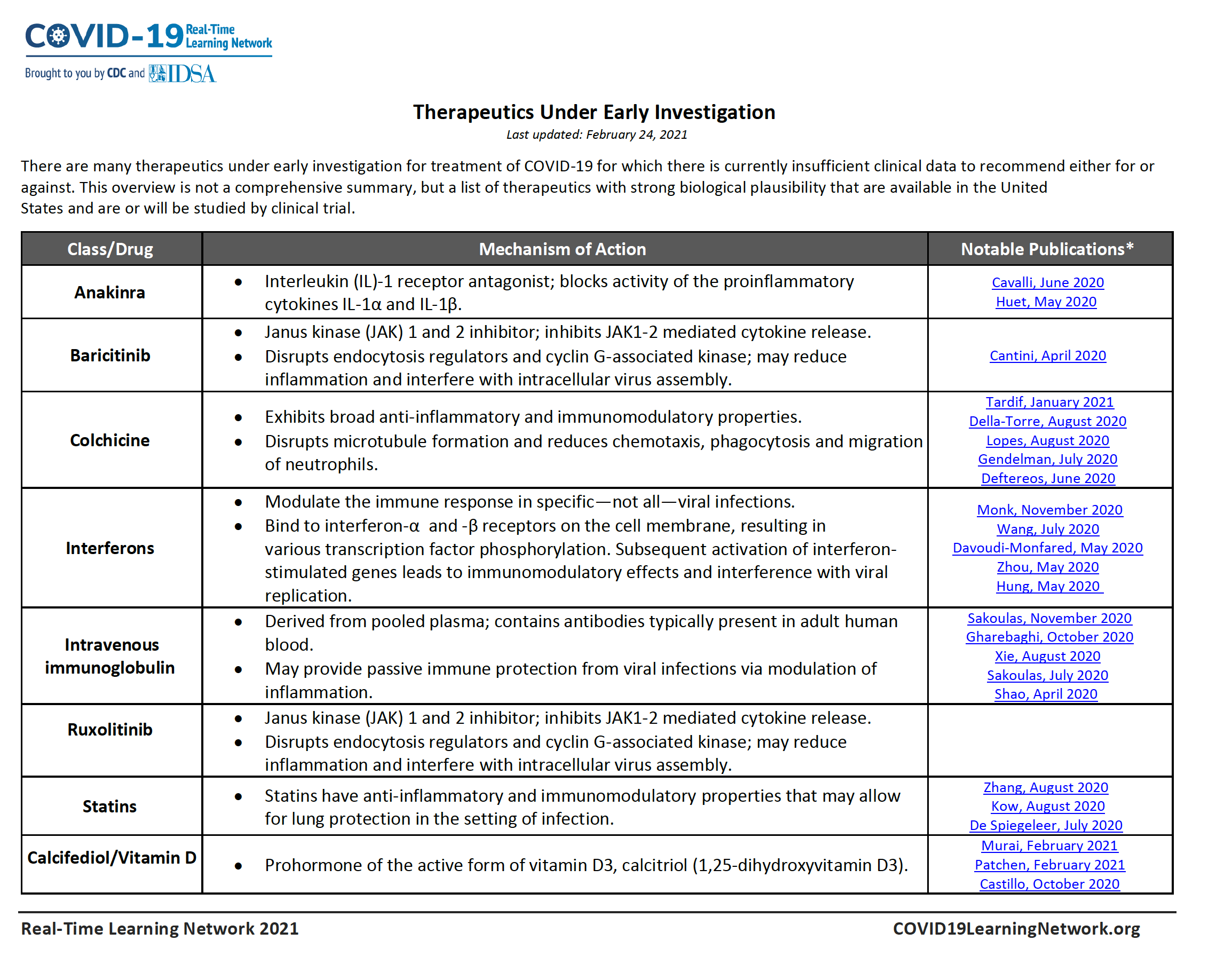
Table of Drugs Under Early Investigation for COVID-19
There are many therapeutics under early investigation for treatment of COVID-19 for which there is currently insufficient clinical data to recommend either for or against. This overview is not a comprehensive summary, but a list of therapeutics with strong biological plausibility that are available in the United States and are or will be studied by clinical trial.